Selecting proper cryogenic vials, or cryovials, is crucial for maintaining sample viability and ensuring safe storage in any laboratory. With advancements in material science and design, cryogenic vials today offer a diverse range of options to accommodate different lab requirements. This blog will guide you through the key considerations for choosing the right cryovials, focusing on material differences, volume capacities, thread designs, and sterility standards.
Table of contents:
Comparing Materials: Polypropylene vs. Polyethylene in Cryovials
Understanding Volume Capacities: From 0.5ml to 5ml Options
Selecting Between Internal and External Thread Designs
Importance of Sterility and Certification Standards in Cryovials
Cryogenic vials are primarily made of polypropylene (PP) and polyethylene (PE), both known for their ability to withstand extreme temperatures. Polypropylene vials are the preferred choice in most laboratories due to their superior chemical resistance and temperature range (-196°C to 121°C). These vials, such as the AMNGENT Traditional Cryogenic Vials available at Zhejiang Rongda Biotechnology, come with the added advantage of medical-grade PP material, ensuring durability and contamination-free storage. Polyethylene, while less common, is sometimes used for less demanding applications. Its flexibility makes it suitable for certain niche scenarios. Ultimately, laboratories must match the material's properties to the storage conditions and the chemical nature of the stored samples.
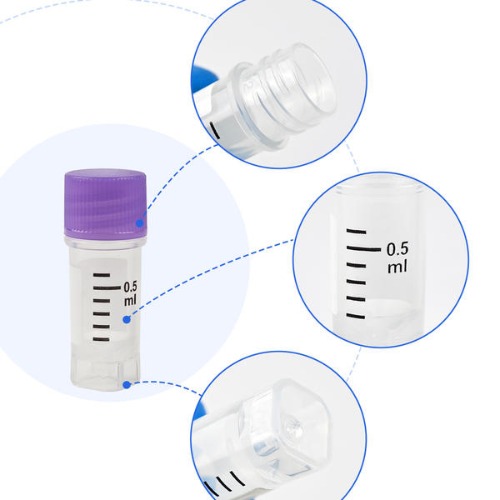
Volume capacity is a critical factor when choosing cryovials. Many traditional cryogenic vials, such as those offered by AMNGENT, are available in volumes ranging from 0.5ml to 5ml. Smaller volumes, like 0.5ml or 1.5ml, work perfectly for applications involving smaller sample sizes and fit into 100-well cryovial boxes. For larger sample storage needs, 2ml and 5ml vials are common options, with 2ml vials often fitting into the standard 81-well cryovial boxes. Laboratories handling a diverse set of sample sizes should consider stocking multiple sizes to accommodate all workflows effectively. For streamlined inventory management, it helps to select vials from suppliers offering multiple volume variations in standardized designs.
Cryogenic vials are available with internal thread or external thread designs. Each has its own advantages, depending on laboratory preferences and requirements. External thread vials are particularly popular due to their enhanced sealing capabilities, as the cap threads are outside the sample chamber, reducing contamination risks. For instance, AMNGENT Traditional Cryogenic Vials come in external thread models equipped with flat caps or concave plugs for better sealing during storage. Internal thread vials are preferred in scenarios where compact storage is required, as they often take up slightly less space. It ultimately comes down to the nature of the samples being stored and whether contamination or spatial efficiency is a higher priority for your lab.
Sterility and certification are essential considerations for ensuring sample integrity during cryogenic storage. High-grade vials should be certified as RNase-, DNase-, and endotoxin-free to prevent chemical contamination. Furthermore, products like AMNGENT Traditional Cryogenic Vials undergo E-Beam sterilization, providing an added layer of protection. Certification adherence to standards such as ISO9001, ISO13485, and FDA ensures quality and compatibility with global lab protocols. For sensitive samples like clinical or diagnostic research, verified sterility should not be compromised. Choosing well-certified cryogenic vials secures both long-term sample preservation and regulatory compliance.
In conclusion, selecting the right cryogenic vials is an essential step in ensuring the integrity and reliability of sample preservation. By prioritizing sterility, certification, and adherence to global standards, researchers and laboratories can confidently safeguard their valuable samples. High-quality cryogenic vials not only enhance the accuracy of scientific results but also contribute to maintaining compliance with rigorous industry regulations, making them an indispensable tool for modern research.