Selecting the right cryovials is essential for laboratories and biobanks aiming to preserve biological samples under extreme conditions. A reliable cryovials supplier plays a pivotal role in ensuring the availability of high-quality products that meet stringent scientific requirements. Among various options, the AMNGENT Traditional Cryogenic Vials, including the 0.5ml size, stand out for their versatility and durability. These vials are designed to withstand temperatures ranging from -196°C to 121°C, making them suitable for long-term nitrogen canister storage. Choosing the ideal cryovials involves understanding material specifications, quality assurance processes, supplier comparisons, and logistics considerations. This article delves into these aspects to guide procurement professionals and researchers in making informed decisions when sourcing cryogenic storage solutions.
Table of contents:
Understanding Material Specifications in Cryovials for Optimal Storage
Quality Assurance Practices Every Cryovials Supplier Should Follow
Comparing Cryogenic Vials from Different Suppliers: What to Look For
Logistics and Delivery Considerations When Sourcing Cryovials 0.5ml
Material quality is a fundamental factor in the performance of cryovials. The AMNGENT Traditional Cryogenic Vials are manufactured from medical-grade polypropylene (PP), a material known for its chemical resistance, durability, and biocompatibility. This ensures that samples remain uncontaminated and stable during storage. The cryovials come in various volumes, including 0.5ml, 1.0ml, 1.5ml, 2.0ml, and 5.0ml, providing options suitable for different sample sizes and storage box configurations. For example, the 0.5ml and 1.5ml vials fit standard 100-well cryovial boxes, while the 2.0ml size is compatible with 81-well boxes. The caps are available in multiple colors such as red, yellow, blue, green, white, purple, and orange, facilitating easy sample identification. Additionally, these vials undergo E-Beam sterilization and are certified RNase & DNase free and endotoxin free, ensuring the highest purity standards. Their design includes external or internal threads and various cap styles like flat caps, concave plugs, and automation covers, tailored to specific laboratory needs.
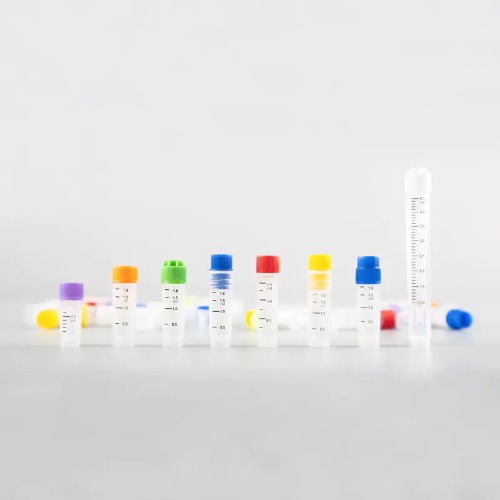
High-quality cryovials must undergo rigorous quality assurance to guarantee their reliability in critical applications. A reputable cryovials supplier like Zhejiang Rongda Biotechnology Co., Ltd., the manufacturer of AMNGENT Traditional Cryogenic Vials, adheres to international standards such as ISO9001 and ISO13485. These certifications underscore their commitment to quality management and medical device manufacturing excellence. The cryovials also comply with FDA and CE regulatory requirements, providing assurance of their safety and efficacy. Quality control processes include verifying material purity, dimensional accuracy, and sterilization effectiveness. Each batch is tested to confirm the absence of RNase, DNase, and endotoxins, which could compromise sample integrity. Packaging is designed to maintain sterility, typically supplied in 50 pieces per bag and 20 bags per carton for most vial sizes, with larger quantities available for specific models. These practices ensure that laboratories receive dependable cryogenic vials capable of preserving sensitive biological samples without degradation.
When evaluating Cryogenic Vials from various suppliers, several factors must be considered to ensure optimal performance and cost-effectiveness. Material composition is paramount; polypropylene remains the preferred choice due to its robustness and inertness. The temperature range supported by the vials should cover ultra-low temperatures, ideally from -196°C up to 121°C, to accommodate different storage environments. Sterilization methods and certifications are also critical; suppliers offering E-Beam sterilized, RNase & DNase free, and endotoxin-free vials provide added confidence in sample safety. The compatibility of vial sizes with standard cryovial boxes and the availability of color-coded caps contribute to efficient sample management. Packaging options, including bulk or smaller quantities, influence procurement flexibility. Moreover, suppliers with proven quality assurance systems and international certifications, such as those held by AMNGENT's manufacturer, typically deliver more reliable products. Considering these criteria helps laboratories select Cryogenic Vials that meet both scientific and operational demands.
Efficient logistics and timely delivery are crucial when sourcing 0.5ml cryovials to maintain laboratory workflows and sample integrity. A dependable cryovials supplier ensures that products are packaged securely to prevent damage during transit, with sterile conditions preserved. Zhejiang Rongda Biotechnology Co., Ltd. provides packaging configurations tailored to customer needs, such as 50 pieces per bag and 20 bags per carton, facilitating inventory management and minimizing contamination risks. Shipping methods should support temperature-controlled environments if necessary, especially for large orders destined for international locations. Additionally, suppliers with experience exporting to over 80 countries, including the United States and European Union, often have established logistics networks that streamline customs clearance and reduce lead times. Clear communication regarding order status and tracking enhances transparency. Considering these logistics factors helps laboratories avoid delays and ensures the continuous availability of AMNGENT Traditional Cryogenic Vials for critical sample storage.
In summary, selecting the ideal 0.5ml cryovials requires a comprehensive understanding of material specifications, stringent quality assurance, supplier reliability, and logistics management. The AMNGENT Traditional Cryogenic Vials, manufactured by Zhejiang Rongda Biotechnology Co., Ltd., exemplify these qualities with their medical-grade polypropylene construction, extensive certifications, and versatile design options. Their compatibility with standard cryovial boxes and availability in multiple cap colors enhance sample organization. Furthermore, the supplier’s adherence to international quality standards and efficient delivery systems ensures that laboratories worldwide receive reliable and sterile products. By focusing on these key aspects, procurement specialists and researchers can confidently source cryogenic vials that safeguard valuable biological samples under extreme conditions, supporting advancements in bioscience and medical research.